CUHK CTC
The Chinese University of Hong Kong Clinical Trials Centre (CUHK CTC) provides pharmaceutical sponsors and investigators with a fully integrated, comprehensive solution for clinical trials in Hong Kong and the Greater Bay Area. As a centralised platform, CUHK CTC streamlines the entire clinical trial process, encompassing investigator identification, regulatory submissions, budgeting, contract negotiation, study execution, and sample archiving.
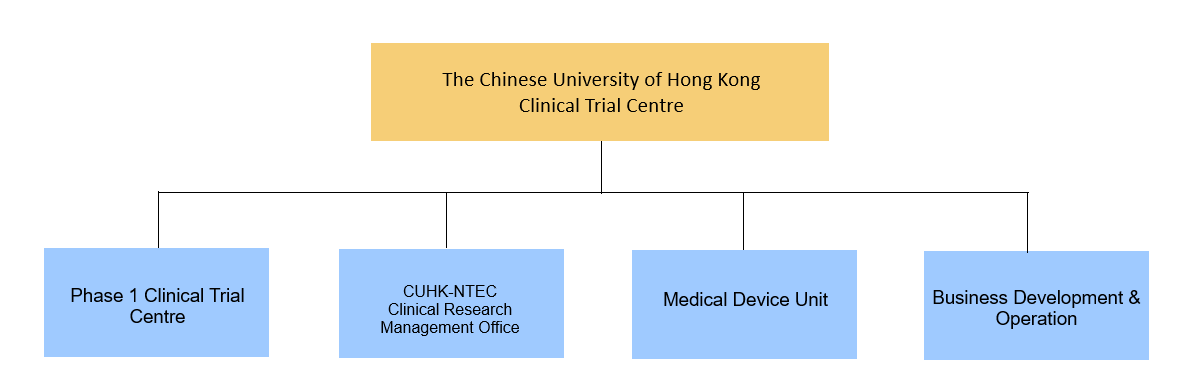
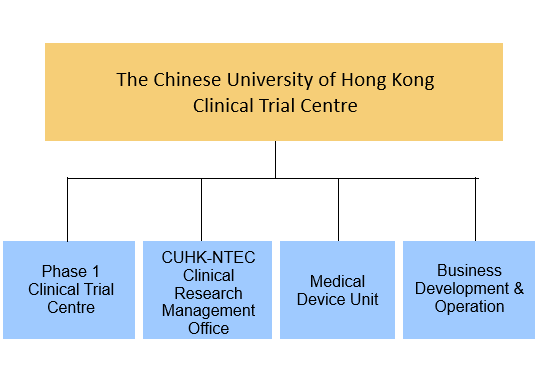
Comprising three core units — the CUHK-NTEC Clinical Research Management Office (CRMO), the Phase 1 Clinical Trial Centre (P1CTC), and the Medical Device Unit (MDU) — CUHK CTC offers extensive support across all phases of pharmaceutical and medical device development, including early-phase and first-in-human studies. Its unified infrastructure ensures accelerated timelines, strict regulatory compliance, and consistent quality, positioning CUHK CTC as a trusted partner for industry sponsors and healthcare providers seeking reliable and efficient trial execution.
Orgainzational Structure